

Chemistry only
Recall that both water and oxygen are needed for a metal to corrode. If we block either the water, the oxygen or both, the metal will not rust. The main methods used to prevent corrosion fall into three categories:
Let's start by looking at different barrier methods used to prevent corrosion.
Barrier methods of preventing corrosion rely on some sort of
barrier to block air/oxygen and/or
water; which are both necessary for
corrosion. For example cars
and bikes are both painted which effectively blocks both air/oxygen and water
from the surface of the metal.
Metal fences for example are often
plastic coated
which again puts up a barrier to air and water.
The problem with some barrier methods are once the
barrier is broken then
the metal will corrode.
Painting and plastic coating are also not suitable for moving metal parts e.g. motorcycle and bike chains, engines and tools such as spanners,
hammers etc. Here the moving parts maybe covered with a thin layer of oil or grease to help prevent corrosion. This is outlined in the images below:
Electroplated objects are metal items that have been coated with a thin layer of another metal. This process, called electroplating, is used to make objects more attractive and to help them resist corrosion. Many car, motorcycle, and bicycle parts are electroplated with chromium metal to give a shiny finish and provide protection against corrosion. Engine parts, exhausts, mirrors, and bumpers are common examples. Inexpensive or costume jewellery is often made from a cheaper metal and then coated with a thin layer of gold or silver to improve appearance and prevent tarnishing. Similarly, cutlery may be electroplated with silver — an unreactive metal that resists attack by acids found in certain foods. The image below shows common everyday objects that are electroplated.


Tin cans that hold a wide range of foodstuffs are actually made from the alloy steel, not the metal tin. Most steel cans have a thin layer of the less reactive metal tin coating the inside surface. Because tin is an unreactive metal, it resists corrosion and attack by acids in the food much better than the steel can itself. The cans therefore benefit from the strength of the steel and the chemical resistance of the tin The electroplated tin cans are manufactured by first forming the steel into the desired can shape. The steel is then cleaned and coated with a thin layer of tin by a process called electroplating.
This involves immersing the steel can in a solution containing tin ions and then applying an electric current. A thin, protective layer of tin is deposited onto the surface, forming a durable tin coat that makes use of tin’s low reactivity. Many modern steel cans are also lined with a thin plastic coating for extra protection, preventing direct contact between the food and the metal and reducing the risk of unwanted chemical reactions or contamination.
However, if the can is dropped or damaged so that the protective tin coating is broken, the exposed steel will rapidly corrode, spoiling the food inside. A more reactive metal connected to a less reactive metal will “sacrifice” itself to protect the other from corrosion. This principle is used to help slow down the corrosion of metals; this is detailed in the paragraph below on sacrifical protection:

A more reactive metal can be used to help prevent a less reactive metal from corroding. Recall that when a metal corrodes, it is oxidised; that is it loses electrons. However; if this metal is in contact with a more reactive metal it will not be oxidised and therefore will not corrode. The more reactive metal will be oxidised instead. The more reactive metal loses electrons to the less reactive metal and prevents it from being oxidised or corroded. In effect, the more reactive metal sacrifices itself to protect the less reactive one. This method of corrosion prevention is called sacrificial protection for obvious reasons.
Zinc is a more reactive metal than iron. When a
metal item, usually one made from steel is galvanized it is dipped into a bath of molten zinc or is electroplated using a zinc solution.
Either way this coats the metal item with a thin layer of zinc metal. This layer of zinc prevents the iron/steel from
corroding.
However if the zinc layer is scratched or damaged and the iron exposed to air and oxygen it will simply corrode. However the more
reactive zinc will sacrifice itself and slow the
corrosion of the iron.
When iron rusts it forms Fe3+ ions by losing 3 electrons, the equation for this oxidation reaction given below:
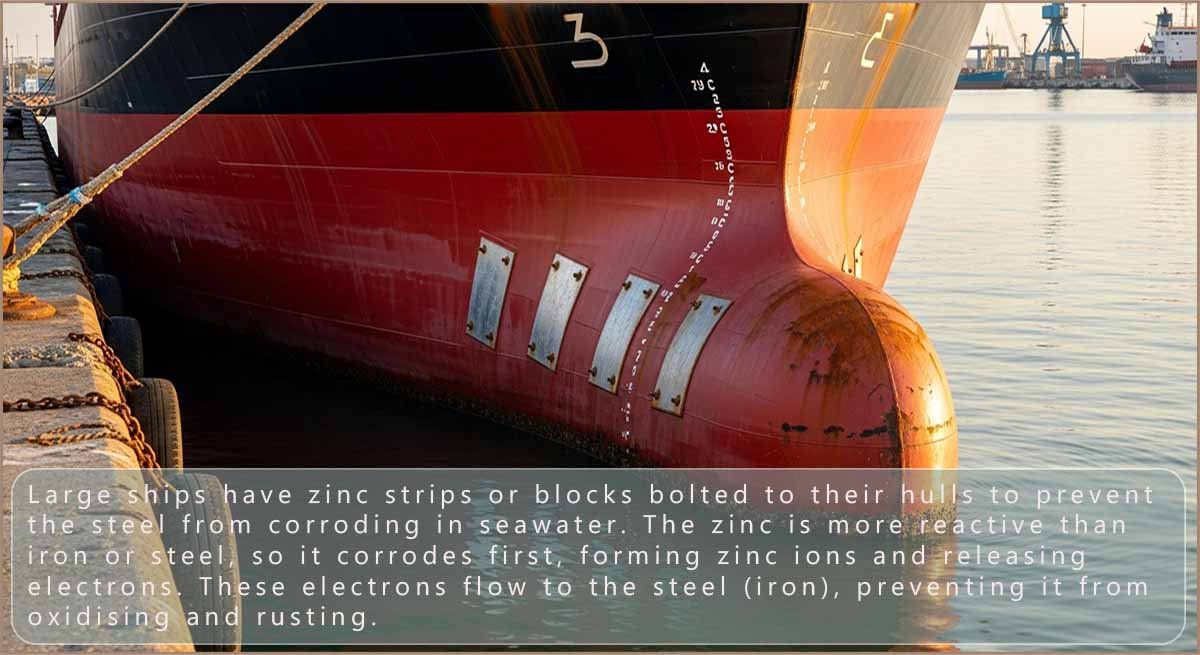
Cars are zinc coated or galvanised to prevent corrosion, this means that even if the paint is chipped and the steel is exposed to air and water no corrosion will occur. Boats, sea piers and oil rigs also have a similar method of corrosion protection. Boats have large pieces of zinc plate bolted to the underside of their hulls and oil rigs and sea piers have long zinc strips attached to their legs. These zinc strips help prevent corrosion by sacrificing themselves and corroding to protect the steel which makes up the boats, pier legs and oil rigs. Periodically these zinc blocks will have to be replaced with fresh blocks as they slowly corrode away.

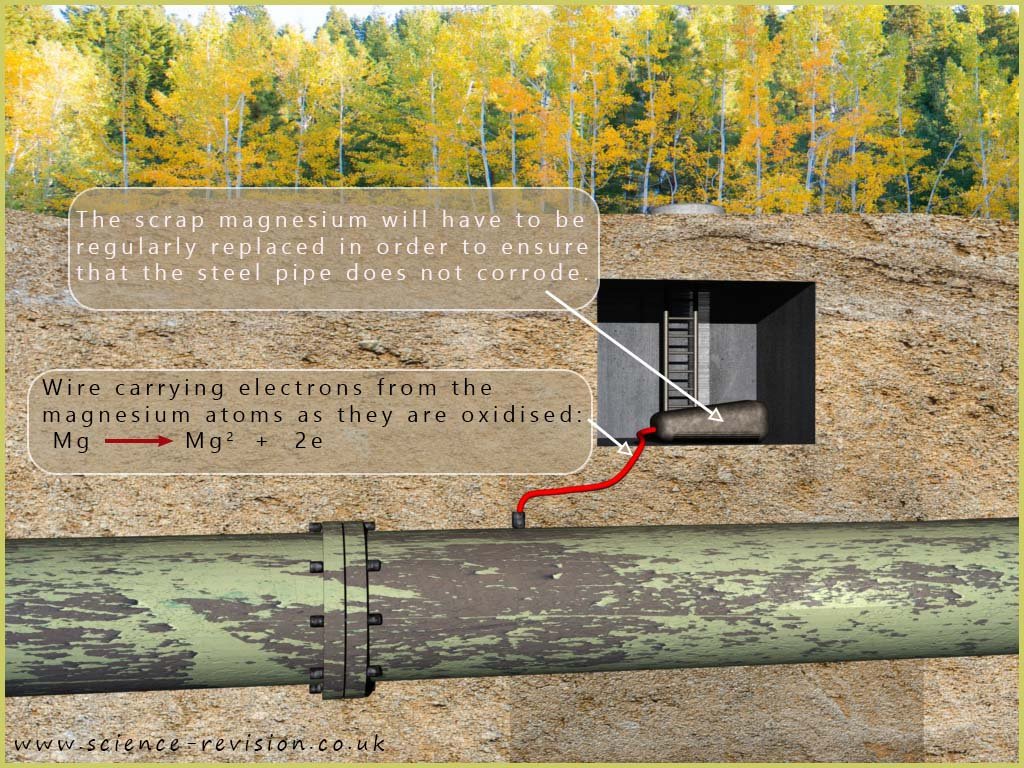
It is not necessary for an object to be completely coated with a more reactive metal in order to protect it from corrosion, as is the case with galvanising. As long as the two metals are in contact, corrosion can be prevented. For example, underground steel pipes can be protected from corrosion by connecting them with a wire to pieces of scrap magnesium, a more reactive metal. The magnesium will corrode (be oxidised) and release electrons that flow through the wire to the steel pipe, preventing it from corroding. This is an example of sacrificial protection, also known as cathodic protection. The image below shows an underground steel pipe protected in this way.
Alloys are mixtures of metals and occasionally non-metals. Mild steel for example is an alloy made by mixing 99.5% iron with 0.5% carbon. The small amount of carbon makes the iron harder and stronger. Mild steel's strength makes it a valuable material for bridge building, construction, and motor car bodies. However, mild steel is still prone to corrosion. However a new alloy of iron can be made by mixing it with a small amount of carbon and also the transition metals chromium (20%) and nickel (10%), this new alloy is called stainless steel. Stainless steel is much harder and stronger than mild steel and it also has the added advantage that it does not corrode, however it is very expensive to produce. Brass (70% copper, 30% zinc) and bronze (90% copper, 10% tin) are two other alloys that are corrosion resistant. They are used to make items such as statues, monuments, and musical instruments.
Complete the activity below by simply matching the corrosion prevention methods with a real-life example of that method being used.
Click one item from each column to make a pair, then press Check answers.